Introduction
The artificial urinary sphincter device has been around since the 1970s. The design is so good that it is still considered the gold standard operation for moderate to severe stress incontinence, sustained in men after treatment for prostate cancer (see post prostatectomy urinary incontinence). This type of incontinence occurs during physical exertion such as coughing, laughing, or exercising. It is due to weakness of the urinary sphincter (a ring of muscle under the bladder that acts like a tap). This surgery has high success rates with long-term durability. It is also very rarely done in women with refractory stress urinary incontinence.
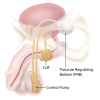
It consists of 3 components that are interconnected:
- A pump that is implanted in the scrotum.
- An inflatable cuff around the urethra.
- A balloon reservoir that is implanted in the lower abdomen.
At all times, the cuff is inflated and keeps the urethra (the passage that drains the bladder) closed. This prevents leaking during normal day-to-day activities and also during physical exertion. During urination, the pump is squeezed to move fluid out of the cuff that is compressing the urethra, and back into the reservoir. This allows urination to occur. After 1-2 minutes, the fluid automatically returns from the reservoir to the cuff, squeezing the urethra closed again.
Indications
Moderate to severe stress urinary incontinence can occur in men after:
- Prostate surgery for cancer.
- Radiotherapy for prostate cancer.
- Prostate surgery for benign prostatic enlargement (e.g., TURP).
- Pelvic trauma that damages the urinary sphincter.
- Neurogenic conditions that weaken the urinary sphincter.
This surgery is not suitable for those with:
- Poor hand dexterity or mental capacity to know how to use the device.
- High anaesthetic risks.
- Known allergy to rifampicin, minocycline or other tetracyclines (antibiotics impregnated into the device).
- Unresolved overactive bladder or poor bladder compliance (‘stiff’ bladder that does not stretch) – because of the risk of building up pressure in the bladder by closing off the urethra, causing damage to the kidneys.
- Known bladder neck contracture (scarring and narrowing), that requires repeated endoscopic procedures to treat.
- Patients who are doing intermittent self-catheterisation.
Preoperative Instructions
If you are taking blood thinners or certain newer diabetic medications, please inform your doctor as these may have to be stopped before the surgery.
You will need to fast for at least 6 hours before the surgery.
Your doctor may require you to have blood tests or a urine test prior to the surgery.
See preparing for surgery, for more detailed instructions.
Procedure
The surgery is usually done under general anaesthesia and takes about 1.5 to 2 hours. Antibiotics are administered through a drip. Prolonged prepping and washing of the surgical field are done. A small catheter is placed. Two skin incisions are made. The first is at the perineal area (area between the scrotum and anus). This allows access down to the urethra, which is freed up. The circumference of the urethra is measured, and an appropriate-sized cuff is then prepared.
A second lower abdominal incision (either left or right side) is then made. This allows the placement of the balloon reservoir and the pump in the scrotum.
Once the cuff is placed around the urethra, all three components are connected with special tubings.
A cystoscopy is done to check for any potential injury to the urethra, and also to check the co-aptiveness of the cuff. A small catheter is then placed in the urethra. The wounds are closed, and dressings are applied. The AUS device is deactivated (switched off).
Postoperative Instructions
Most patients stay in the hospital for 2 days. A catheter is placed in the penis to help drain the bladder overnight post-operatively. This is removed the following morning after surgery on day 1. You are encouraged to mobilise around the ward and do deep breathing exercises to prevent clots in the legs and chest infections respectively.
Importantly, the AUS device must be deactivated (switched off) for the first 6 to 8 weeks after the surgery. This is because the swelling and discomfort in the scrotum will prevent you from using the pump during the recovery period. Therefore, you will still be incontinent and must keep using pads.
You will receive intravenous antibiotics for 2 days during your stay in the hospital. For discharge, you will be given oral antibiotics for about a week to prevent infection of the device. Inform your doctor if the wounds have an offensive discharge or if you develop a fever at home.
You are expected to get some scrotal swelling and bruising after the surgery. This is due to the rich blood supply in the area. Wear supportive underwear (not boxers) to reduce the discomfort. Take your pain medication as directed.
Your doctor will give you instructions on when you can restart your blood thinning medications. This instruction will vary depending on the type of medication, the reason why you are on it, and the degree of bruising you get post-operatively.
You can do light duties at home including walking up 1-2 flights of stairs, going for short walks, and going to the shops (but no heavy lifting) in the first few weeks. Slowly increase your activity level as you become more comfortable.
Remove the dressings in 5 to 7 days’ time. Do not worry if they fall off before then. Just keep the wound clean. The dressings are waterproof, and you can shower with them. The skin sutures are dissolvable and take a few weeks to do so.
Do not drive for at least 2 weeks as you may still have some post-op pain.
Check with your doctor when you can go back to work as the type of work that you do will determine this. In general, if your work is not too physically demanding, you can go back to work in 3 to 4 weeks. If it is more demanding, you may need about 6 weeks off work.
Attend the post-op review at the 6 to 8 week mark, so your doctor can check your wounds, activate, and teach you how to use the device. You will be continent after that.
Occasionally, you may still get mild drips of leakage when getting up from sitting on a hard surface. This occurs when urine slips through the cuff area during the split second as the cuff is trying to reinflate itself, after being compressed against the hard surface.
In the long term, patients and their family members have to be aware of the presence of the AUS. In particular, patients are not allowed to:
- Have a catheter inserted into the urethra without deactivating the AUS. This will cause erosion (tearing and injuring) of the urethra where the cuff is, and lead to device failure, infection and even sepsis (severe infection in the bloodstream). The AUS will then have to be removed entirely. In case of a medical emergency or when preparing for other elective surgery during which a catheter is anticipated, please contact your urologist for help.
- Have any injections near where the balloon reservoir is in the lower abdomen and along the path of the tubings down to the cuff and pump. Some medical procedures involve puncturing the groin blood vessels (e.g., angiogram) and may potentially pierce the AUS. This will cause loss of fluid from the system and device failure. It can also cause infection of the AUS, needing its removal.
Risks
General risks:
- Anaesthetic risks such as heart or lung problems.
- Wound infection – you will be covered with antibiotics during the surgery.
- Bleeding – it is uncommon to need a blood transfusion for this surgery.
- Clots in the legs (DVT) or pulmonary emboli.
- Chest infection, urinary tract infection.
- Allergic reactions (e.g., to dressings, drugs etc.) – inform us if you have any known allergies.
- Risk of death is a very rare complication that may arise from any surgery or anaesthetic. Modern medicine and anaesthesia have made this extremely rare. The risk varies with each individual’s general health conditions and the complexity of the surgery. The artificial urinary sphincter surgery is not considered to be a major surgery.
Specific risks:
- Urethral injury can occur rarely during the surgery. If it is significant, the urethra will be repaired, and the AUS surgery will be aborted.
- Urethral or cuff erosion: this is a dreaded complication that urologists fear. It is uncommon. The cuff can cause a tear in the lining of the urethra and make a hole. When this happens, the device malfunctions and the patient may describe blood in the urine, pain, or fevers. The whole AUS must be removed then to allow the urethra to heal. The risk of erosion is higher in patients who had radiotherapy or previous urethral surgery. Procedures involving the insertion of instruments or catheter in the urethra can also cause this, if the AUS is not deactivated just prior. See below for A/Prof Gani’s publication on his technique to reduce the risk of cuff erosion in high-risk patients.
- Urethral atrophy: after many years (usually > 10 years) of constant compression of the urethra, its circumference may shrink. This will then cause the cuff to become loose, and the patient will describe a recurrence of the incontinence. This is uncommon. A revision surgery is usually done to replace the AUS device.
- Device malfunction: the AUS device is very durable, and some patients have no issues after 15 – 20 years. However, any device can develop technical failure in the future. The rate of revision surgery for this is about 10% to 15% in 10 years.
Treatment Alternatives
For treatment alternatives, see post prostatectomy urinary incontinence.
Related Information
A/Prof Gani has extensive experience in the artificial urinary sphincter surgery. He is one of the highest volume implanters of the device in the country. A novel technique that uses a strip of fascia (natural abdominal wall tissue) to wrap around the urethra that acts as an additional protective layer. This lowers the risk of cuff erosion especially in higher-risk patients.
Read A/Prof Gani’s publication:
Download A pilot study of autologous rectus fascial wrap at the time of artificial urinary sphincter placement in patients at risk of cuff erosion - PDF (731 Kb)